Contact us
Industrial wastewater contains a wide variety of contaminants. These can include for example heavy metals from a metal-processing plant, fats and oils from parts cleaning or medicinally relevant substances or ingredients from the pharmaceutical industry - the wastewater is as diverse as the processes themselves. There is a plethora of different industrial wastewater treatment methods on the market - all with their specific strengths and weaknesses. Here is an overview of the most common processes:
In general, industrial wastewater treatment can be divided into two main classes: In one class, problematic substances are removed from the water, e.g., by means of filters/membranes or alternatively by evaporation. In the other class, adjuvants are added selectively to improve water purity and quality. This happens, for example, in chemical purification, which utilizes the addition of acid or alkali to regulate pH or salts to tune conductivity and solubility.
There are four main methods in wastewater treatment:
By far the most commonly used industrial wastewater treatment methods are chemical-physical splitting plants, membrane plants and vacuum distillation systems.
In some cases, there is only one optimal method for the process water to be treated and it is governed by the amount and degree of contamination of the water. If the contaminant load is almost exclusively inorganic, membrane filtration processes can be ruled out because they are too costly. Vacuum distillation is not suitable if the process water contains latex, lacquer, paint or protein. But the following figure also shows that there is a wide range in which all the listed processes are applicable. In this range, the processes must be carefully weighed against each other.
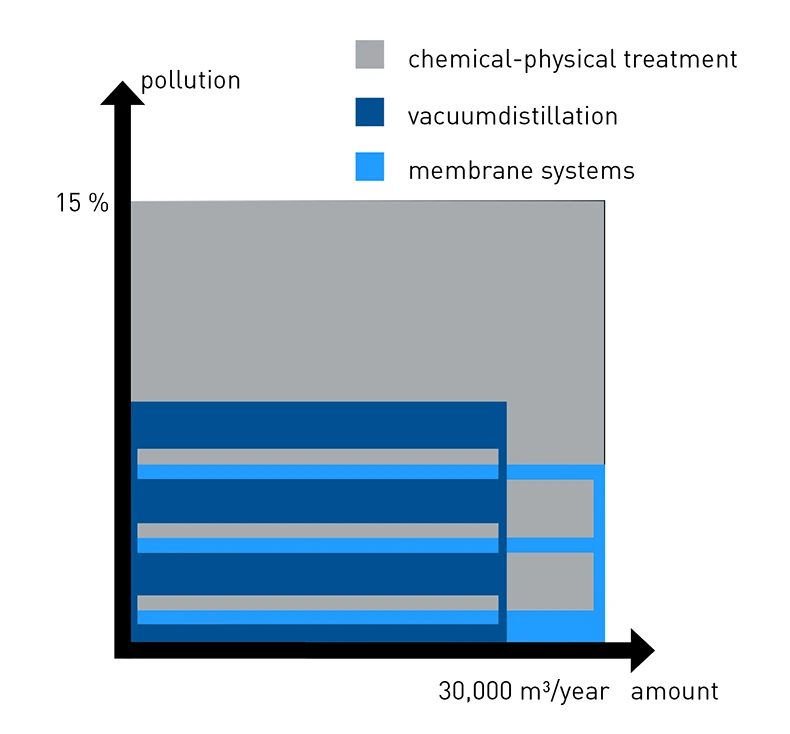
In the area of "relatively low pollution load of up to approx. 7 percent evaporation residue and a wastewater volume of up to 30,000 cubic meters/year", the three wastewater treatment processes overlap. Here it is necessary to prioritize the technologies according to the most important criteria to identify the optimal wastewater treatment system for oneself.
Why should you treat the wastewater in your company and not simply dispose of it or have it treated by public wastewater treatment plants?
Treating water is a complex and expensive process. For the sake of the environment, it is important to discharge as small a volume of wastewater as possible into the treatment process.
Concomitantly, statutory limits and the Wastewater Ordinance determine whether the wastewater can be discharged into public networks.
By using a vacuum distillation system such as the VACUDEST, not only are the limits for discharge met, but also is the volume of wastewater you have to dispose of drastically reduced, thus saving a large part of the costs associated with disposal.
To understand why already a low volume should be targeted not only from a cost perspective but also from an environmental perspective, it is important to be informed about what happens to the wastewater after it leaves your company.
Wastewater treatment is generally conducted in three stages. At first, the process focuses on filtering coarse materials from the water and then using progressively finer methods so that the water gains purity at each stage. The stages are therefore called primary, secondary and tertiary. In some cases, an additional stage is needed after the third, but it shall not be described here. After each stage, different contaminants are removed from the water.
In the first stage, the water is kept stagnant in a basin. This causes solids to settle by gravity at the bottom of the basin, while solids lighter than water accumulate on the surface. After a certain period of time, all these substances are separated.
Mechanical devices ensure that the (still polluted) water between the solids can be drained from the basin into another basin for the second cleaning stage. Oftentimes, the solids are also captured so that further water can be extracted from them.
In the second stage, biological and organic constituents in the water are degraded by aerobic processes. From a chemical point of view, these processes are oxidations and as such rely on the presence of oxygen. Oxidation massively reduces the biological content in the water. The second stage of wastewater treatment allows the treated water to be returned to nature; this means that biological pollutants are not present any more to a harmful extent.
Depending on the pollutants in the water, different technologies can be used in this second stage:
Biofilter
To ensure that all solids are removed from the water, trickling filters, gravel filters or contact filters can be applied. Contact filtration utilizes precipitation chemicals such as aluminum sulfate or ferrous sulfate to remove phosphates from the water. Purification by trickling filters involves spraying the water over a solid material such as lava slag. Bacteria colonizing the lava slag will then break down all biodegradable ingredients.This third stage of wastewater treatment ensures that the quality of the water is high enough to be returned to industrial and domestic uses. The third stage of wastewater treatment usually includes one or more of the following steps:
For a holistic comparison of internal wastewater treatment on the companies’ premises, it is first necessary to define some criteria:
With treatment concepts such as membrane processes or chemical-physical processes, it is more economical to treat the wastewater just enough to make it suitable for discharge. As a result, residual contamination is entering public networks. Treated city water is then used in production because the process costs here are lower than the more extensive treatment of the already treated wastewater. In both categories, quality and sustainability, these two systems do not score so well.
The purchase of a chemical-physical plant is comparatively cheap. However, there are high costs for consumables involved. At the same time, handling a wide variety of chemicals, especially when there is a wide range of ingredients in the wastewater, is labor-intensive and difficult. This technology has more weaknesses, especially when the composition of the process water changes. If necessary, the entire process would have to be adapted to the changed conditions. With regard to the safe separation of heavy metals, this technology shows certain risks. For example, complexed heavy metal compounds can be difficult to separate since they remain in solution throughout the treatment cascade.
Membrane treatment plants have moderate consumption values, but the amounts of residue to be disposed of and thus the remaining disposal costs are high. The purification process for membrane plants must be started manually and the results monitored. Changes in the process can cause blockage of the membrane, so that replacement of the membrane modules might be necessary. Similar to chemical-physical processes, complexed heavy metals may also not be reliably separated here and lead to consent limit values being exceeded.
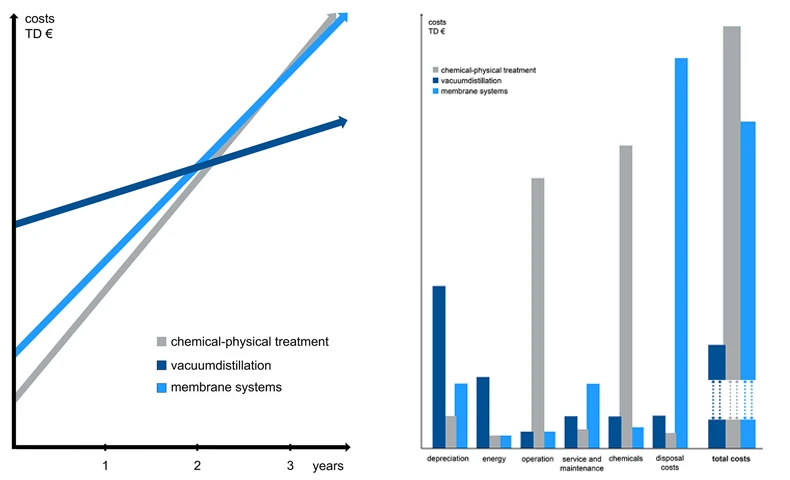
The operating cost comparison of the 3 processes illustrates that the vacuum distillation technology amortizes the higher investment price just after about 2 years through lower operating costs.
If the plant is to become wastewater-free, or if the production processes require very pure rinse water, vacuum distillation is a suitable option. The quality of the distillate is so high that none or few post-treatment steps are necessary. The resulting distillates are almost free of oil and heavy metals. Only if extremely high-quality requirements are imposed on the process water, post-treatment via ion exchangers is necessary. Otherwise, the water can be reused in the company's own production: this saves fresh water, and no residual contamination is released into the environment.
If one considers the investment and operating costs of vacuum distillation plants in the range of 100 - 30,000 m³/year with contaminant loads of less than 8 per cent, the economic advantages of vacuum distillation technology become quite apparent. Although the investment costs are higher than for other processes, the operating costs are unbeatably low because the system works nearly 100 % automatically.
Vacuum distillation also sets the standards in terms of flexibility and safety. Modern systems automatically adapt to fluctuating process water qualities. Minor adjustments allow galvanic process water to be treated tomorrow in a system that was actually designed for the treatment of spent cooling lubricant emulsions.
More information about vacuum distillation here.
You want to be part of our team and create the wastewater-free future with us?
We will tell you how!
Your contact is:
Bettina Böhringer
Human Resources
+49 7627 9239-201
career@h2o-de.com
You have questions on our VACUDEST systems?
Kindly contact us!
Your contact is:
Thomas Dotterweich
Senior Sales Engineer
+49 7627 9239-306
thomasm.dotterweich@h2o-de.com
You need consumables, spare parts or a maintenance date?
We will be pleased to assist you!
Your contact is:
Carles Fité
Technical Customer Support
+49 7627 9239-888
carles.fite@h2o-de.com